|
||||||||||
|
|
||||||||||
|
|
||||||||||
|
Research in the Swihart group centers around the synthesis and application of nanoparticles and the application of chemical engineering science (chemical kinetics, thermodynamics, transport phenomena) to understand and improve processes by which these materials are prepared. Much of our research can be categorized into the six areas listed below. The remainder of this page includes a brief introduction, some relevant images, and some relevant publications in each of the areas listed.
|
|
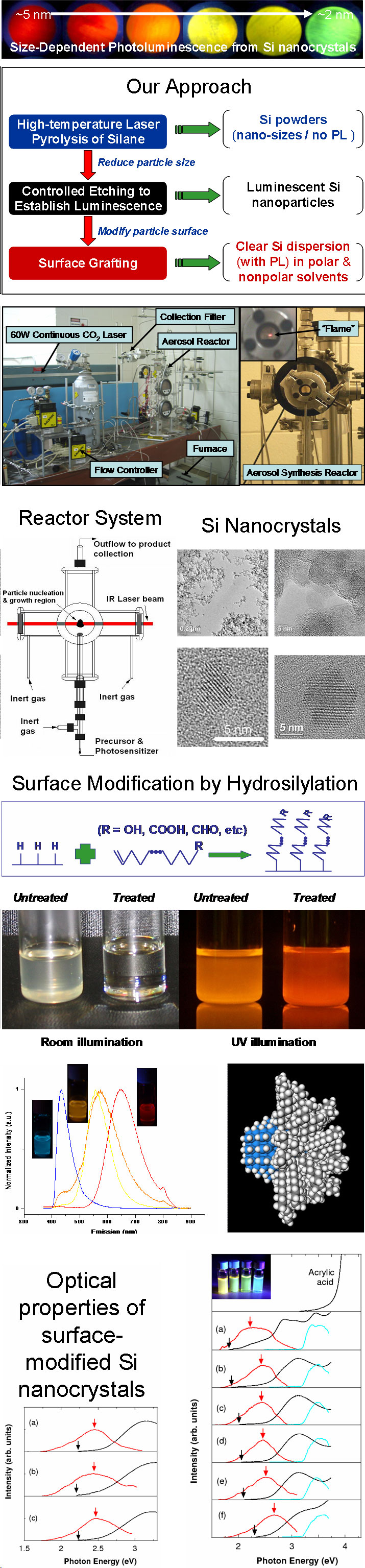
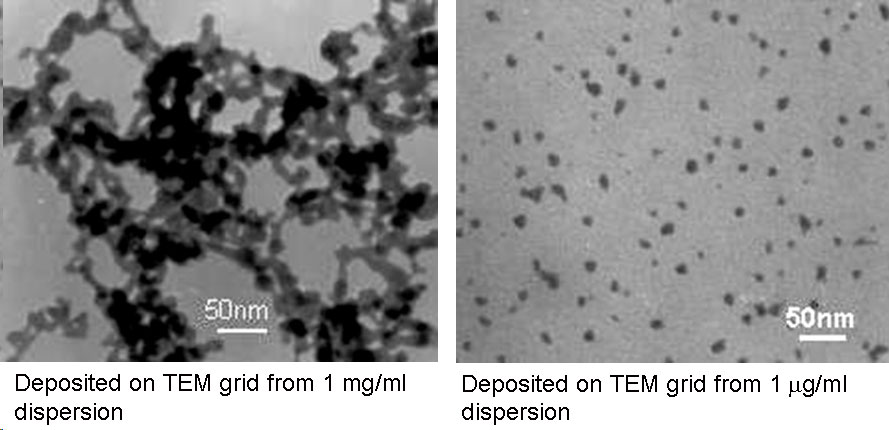
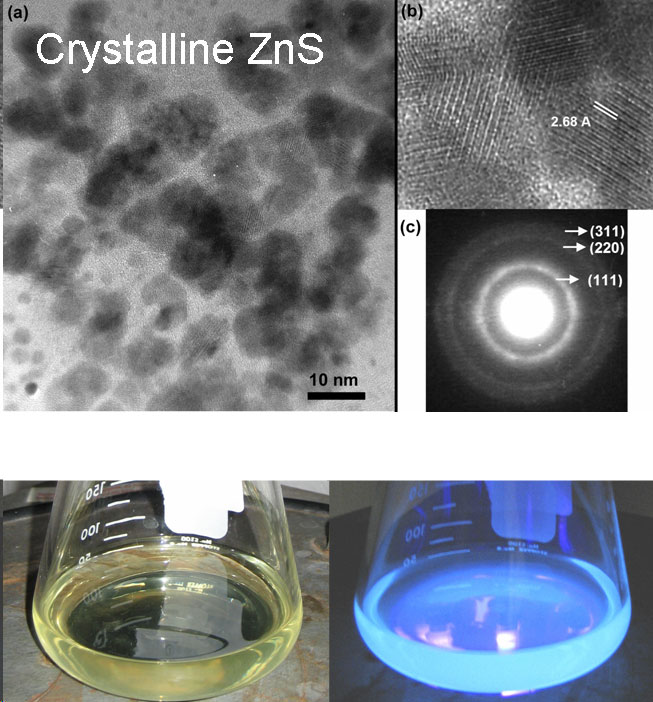
Scalable Synthesis and Surface Modification of Photoluminescent Silicon "Quantum Dots"Brief Introduction:Silicon nanocrystals, like other semiconductor nanocrystals, exhibit size-dependent optical and electronic properties. Despite the fact that bulk silicon is a very poor light-emitter, silicon nanocrystals show efficient emission at wavelengths from the near-infrared to green as their size decreases from about 5 nm to 1.5 nm in diameter. As a result, they have tremendous potential for applications in bioimaging, biomedical assays, solid-state lighting, displays, and photovoltaics. Compared to other semiconductor nanocrystals, they offer potentially important advantages in terms of cost and, especially, low toxicity. However, they are much less amenable to solution phase synthesis than CdSe and other compound semiconductor nanocrystals. Thus, developing improved methods of preparing these nanocrystals and modifying their surfaces is an ongoing research challenge. We have developed a unique, three-step process, illustrated below, that allows us to prepare macroscopic quantities of photoluminescent silicon nanocrystals and modify their surface for subsequent processing and applications. Images:
Related Publications:Sato, Seiichi and Mark T. Swihart, “Propionic acid terminated silicon nanoparticles: Synthesis and optical characterization”, Chemistry of Materials, 18, 4083-4088 (2006). (download PDF)Hua, Fengjun, Folarin Erogbogbo, Mark T. Swihart, and Eli Ruckenstein, “Organically capped silicon nanoparticles with blue photoluminescence prepared by hydrosilylation followed by oxidation”, Langmuir, 22, 4363-4370 (2006). (download PDF) Hua, Fengjun, Mark T. Swihart, and Eli Ruckenstein, “Efficient surface grafting of luminescent silicon quantum dots by photoinitiated hydrosilylation”, Langmuir, 21, 6054-6062 (2005). (download PDF) Kirkey, William D., Yudhisthira Sahoo, Xuegeng Li, Yuanqing He, Mark T. Swihart, Alexander N. Cartwright, Stanley Bruckenstein, and Paras N. Prasad, “Quasi-Reversible Photoluminescence Quenching of Stable Dispersions of Silicon Nanoparticles”, Journal of Materials Chemistry, 15, 2028-2034 (2005). (download PDF) Li, Xuegeng, Yuanqing He, and Mark T. Swihart, “Surface Functionalization of Silicon Nanoparticles Produced by Laser-Driven Pyrolysis of Silane followed by HF-HNO3 Etching”, Langmuir , 20, 4720-4727 (2004).
Li, Zhifeng, Mark T. Swihart, and Eli Ruckenstein, “Luminescent Silicon Nanoparticles Capped by Conductive Polyaniline through the Self-assembly Method”, Langmuir, 20, 1963-1971, (2004). (download PDF)
Kirkey, W.D., A.N. Cartwright, X. Li, Y. He, M.T. Swihart, Y. Sahoo, and P.N. Prasad, “Optical Properties of Polymer-Embedded Silicon Nanoparticles”, Proceedings of the Materials Research Society, 789, N.15.30.1-N.15.30.6, (2004). (download PDF) Li, X., Y. He, S.S. Talukdar and M.T. Swihart, "A process for preparing macroscopic quantities of brightly photoluminescent silicon nanoparticles with emission spanning the visible spectrum", Langmuir, 19, 8490-8496. (download PDF) |
|
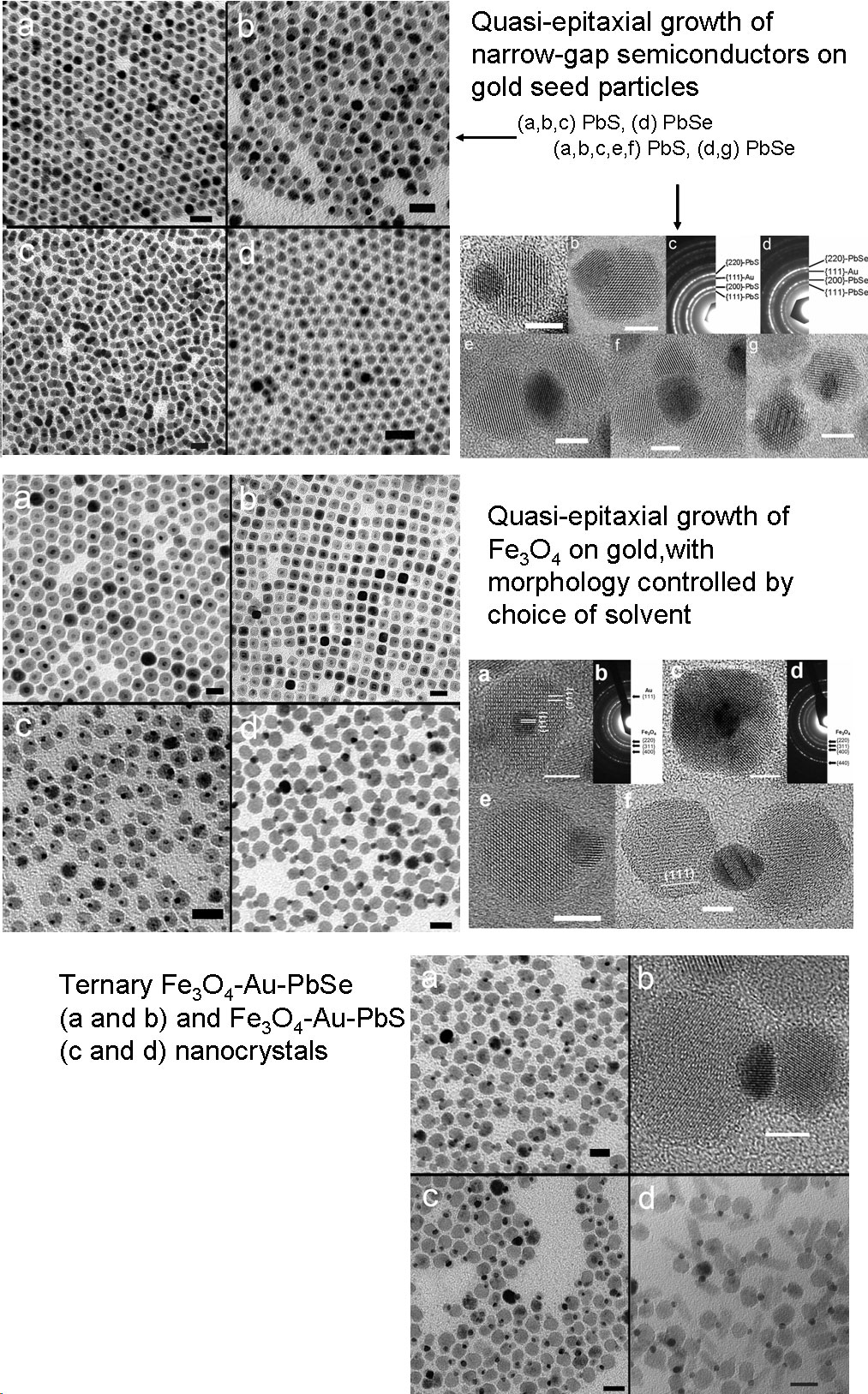
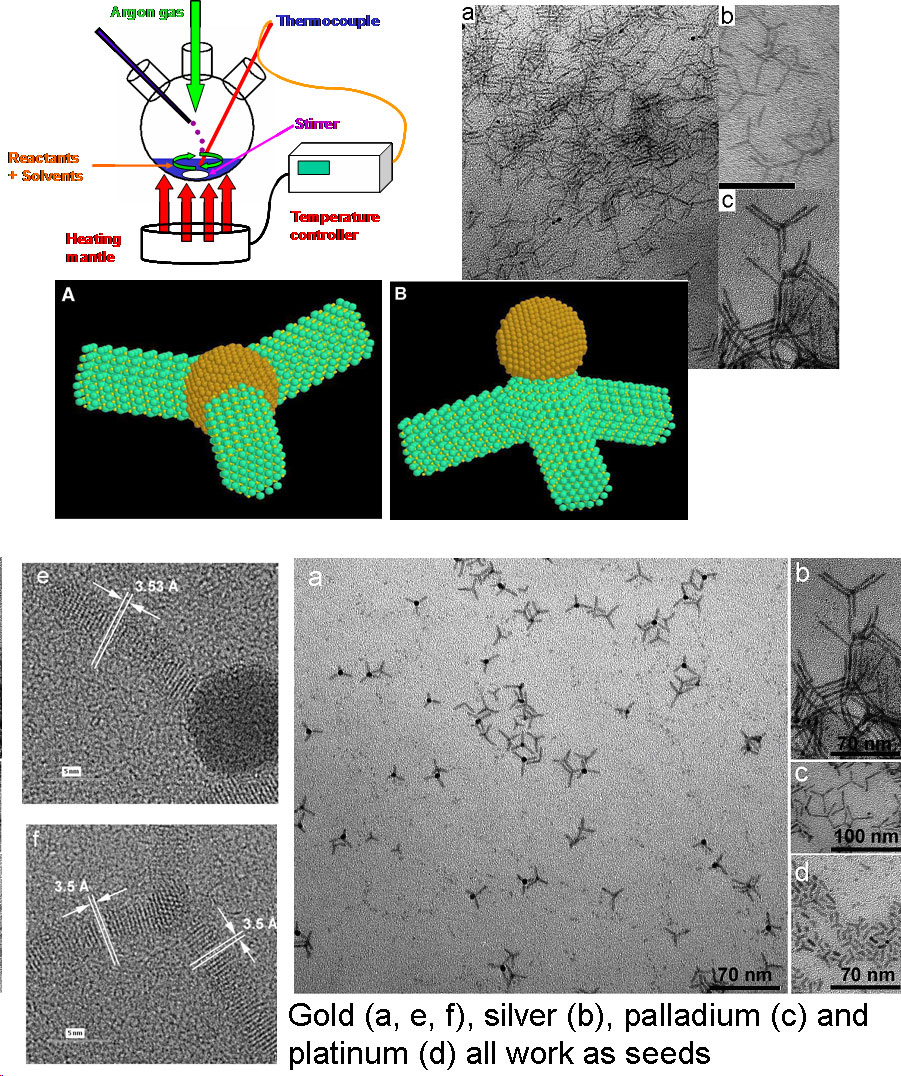
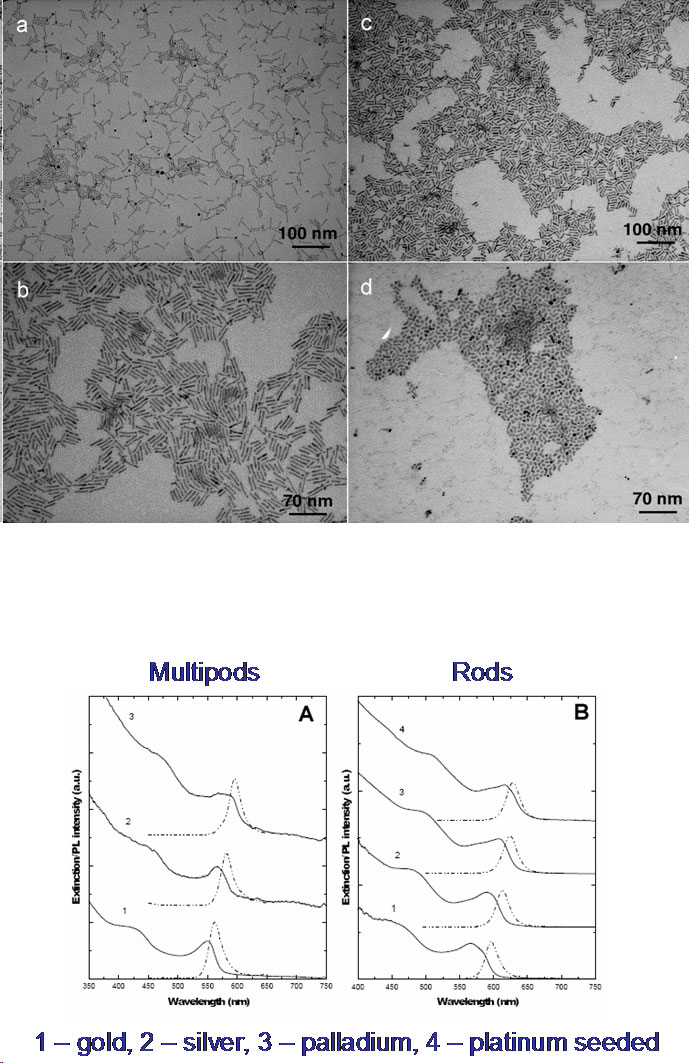
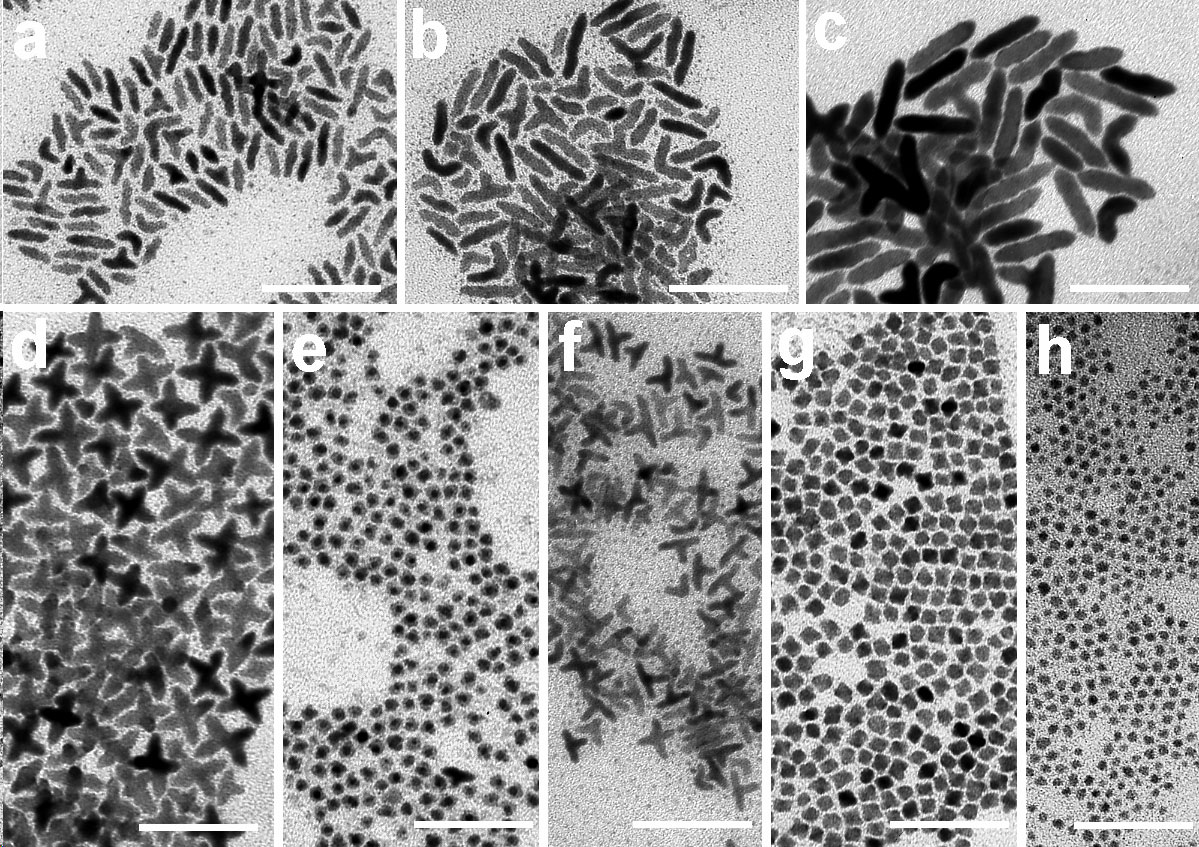
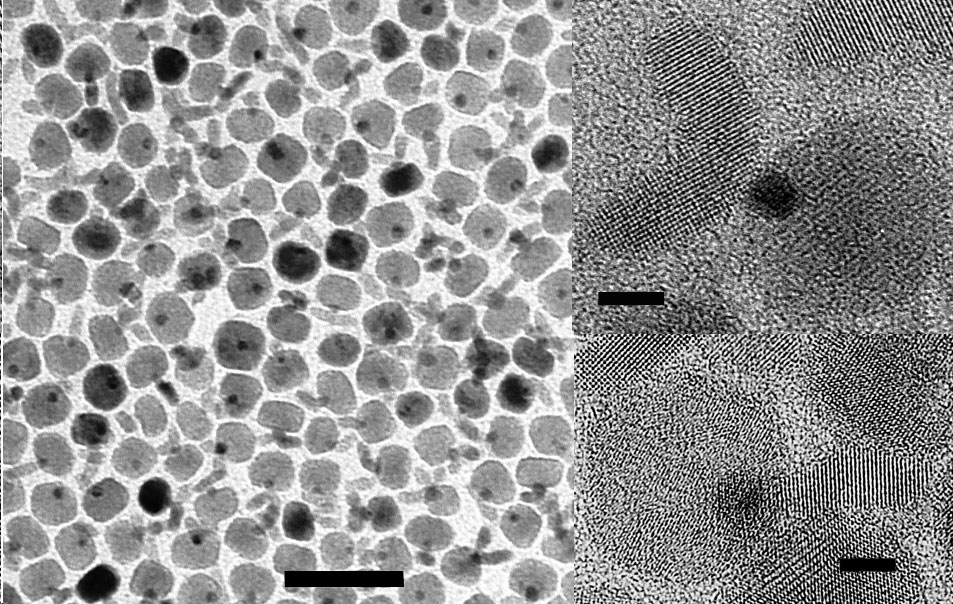
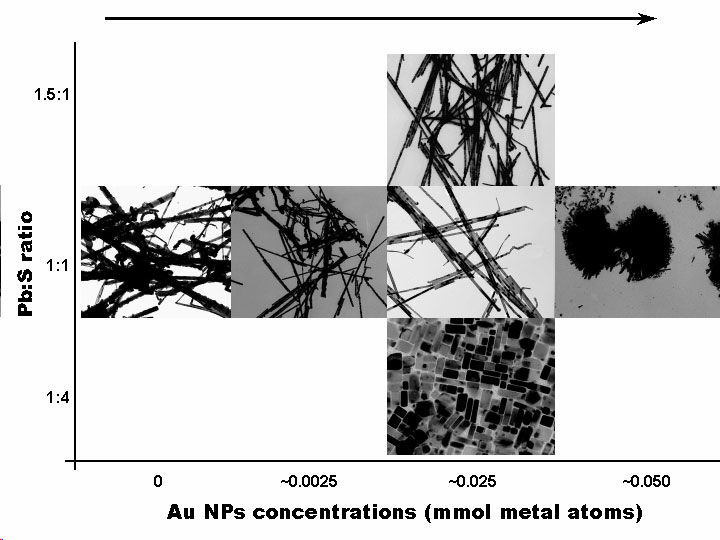
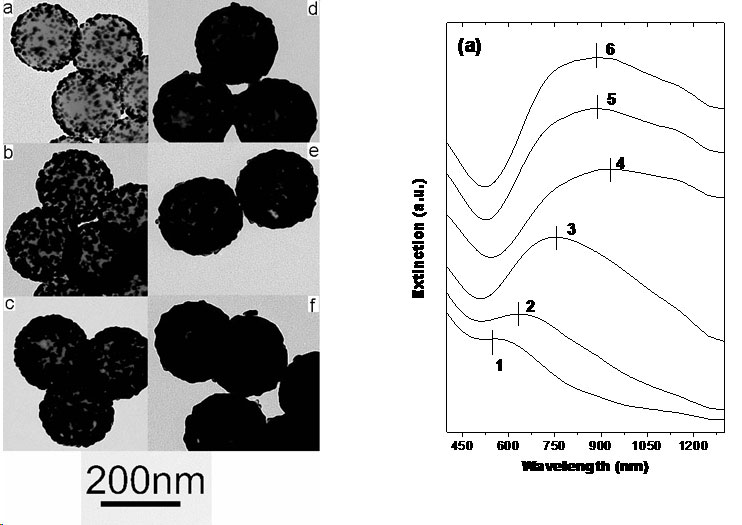
Solution Phase Synthesis of Hybrid and Anisotropic NanoparticlesBrief Introduction:The properties (chemical, electronic, optical, etc.) of nanoparticles depend not only on their size, but also on their shape and arrangement. In general, solution phase synthesis methods offer greater flexibility and control of particle shape than is possible in gas phase methods. In solution, approaches including "seeding" with another material, templating with an existing structure, or modifying the energies of different crystal surfaces using mixtures of surfactants can lead to growth of anisotropic (non-spherical) nanocrystals. Different morphologies can often result from relatively subtle changes in the reaction conditions used. The "seeding" approach, in which a second material nucleates heterogenously on a pre-existing nanoparticle, can allow production of anisotropic nanoparticles, when the growing material eventually falls off the seed particle, or can lead to hybrid nanoparticles in which the "seed" particle and growing material remain joined, with a quasi-epitaxial interface between them. This can even be repeated to form three-component (ternary) hybrid nanoparticles. This allows us to combine different classes of inorganic materials (semiconductors, metals, oxides) each with tunable optical, electronic, magnetic, or chemical (catalytic) properties in unique ways. This has potential for producing new materials with applications ranging from catalysis to photovoltaics to nanomedicine. Images:Quasi-epitaxial growth of binary and ternary hybrid nanocrystals: |
|
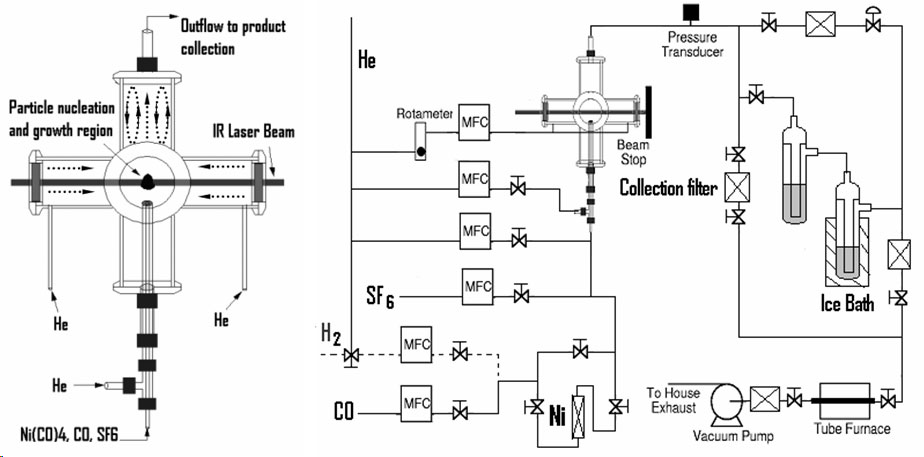
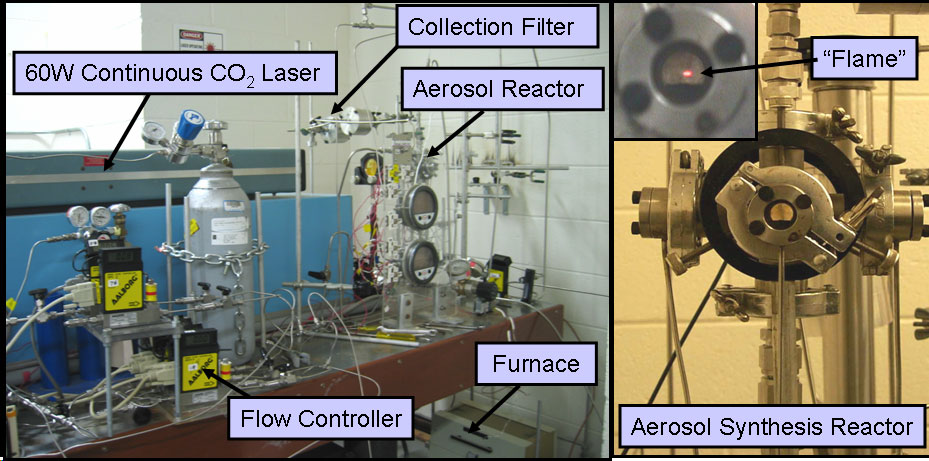
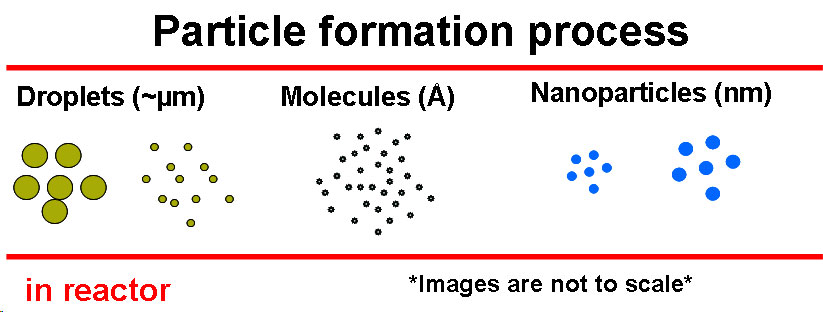
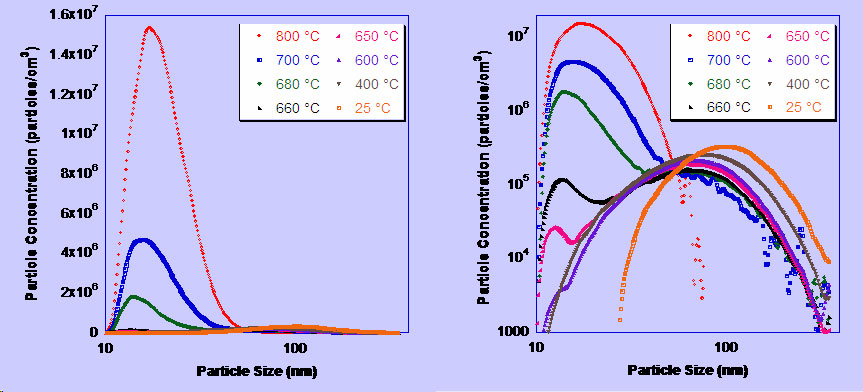
Laser-driven Aerosol Synthesis of Nanoparticles:Brief Introduction:Aerosol (gas-phase) methods of nanoparticle synthesis have some important advantages, including high throughput, production of bare (not surfactant-coated) particles, and elimination of solvents that must be disposed of and that often bring impurities. In a gas-phase process, particles that collide invariably stick together. If they are sufficiently hot (have sufficient energy) they will sinter (partially or completely) to form a larger particle or hard agglomerate of small particles. This is usually undesirable. In order to produce unagglomerated nanoparticles, one must prevent them from colliding until they are sufficiently cool and unreactive that they will not sinter or form hard agglomerates. This requires that either the collision rate be reduced, usually by reducing the particle concentration, or that the residence time during which the particles are hot (reactive) is reduced. Decreasing the residence time for coagulation thus increases the maximum concentration at which particles can be produced. By using a laser to heat a precursor gas, without heating the reactor walls or surrounding gas, we can achieve very rapid heating and cooling rates, and residence times of about a millisecond in the '"reaction zone" where the laser interacts with the gas. This laser-driven synthesis is the first step in our strategy for preparing silicon quantum dots, described at the top of this page. We have also used it to produce nanoparticles of other materials, including nickel, as illustrated here. Images: |
|
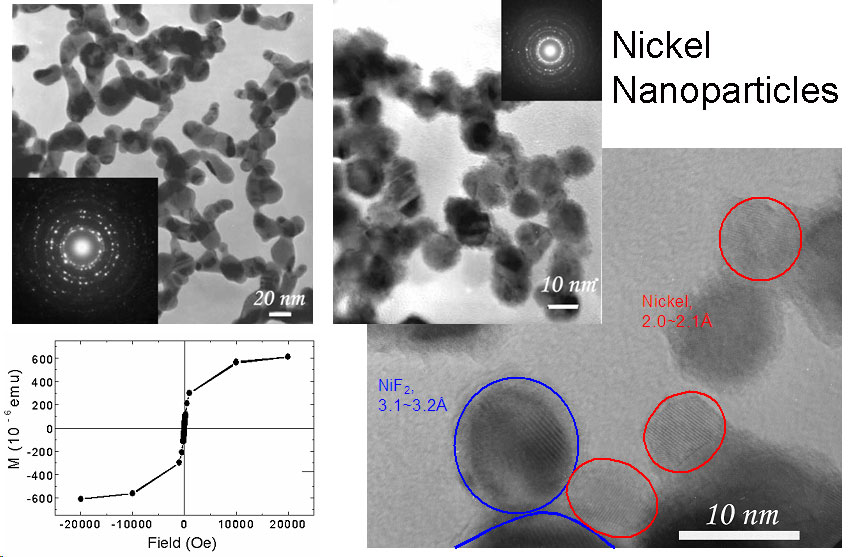
Related Publications:He, Yuanging, Yudhisthira Sahoo, Shumin Wang, Hong Luo, Paras N. Prasad, and Mark T. Swihart, “Laser-Driven Synthesis and Magnetic Properties of Iron Nanoparticles”, Journal of Nanoparticle Research, 8, 335-342 (2006). (download PDF)Sahoo, Yudhisthira, Yuanqing He, Mark T. Swihart, Shumin Wang, Hong Luo, Edward P. Furlani, and Paras N. Prasad, "An aerosol-mediated magnetic colloid: Study of nickel nanoparticles", Journal of Applied Physics, 98, 054308 (2005). (download PDF) He, Yuanqing, Xuegeng Li, and Mark T. Swihart, “Laser-Driven Aerosol Synthesis of Nickel Nanoparticles”, Chemistry of Materials, 17, 1017-1026 (2005). (download PDF) Li, Xuegeng, Yuanqing He, and Mark T. Swihart, “Surface Functionalization of Silicon Nanoparticles Produced by Laser-Driven Pyrolysis of Silane followed by HF-HNO3 Etching”, Langmuir , 20, 4720-4727 (2004). (download PDF) Li, X., Y. He, S.S. Talukdar and M.T. Swihart, "A process for preparing macroscopic quantities of brightly photoluminescent silicon nanoparticles with emission spanning the visible spectrum", Langmuir, 19, 8490-8496. (download PDF) |
|
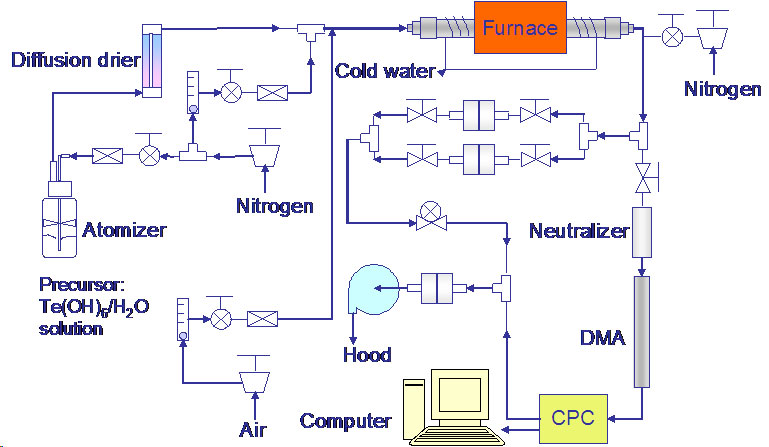
Nanoparticle Synthesis by Spray Pyrolysis:Brief Introduction:In spray pyrolysis, a solution of a precursor compound is sprayed as small droplets. This aerosol of droplets is heated and/or diluted, leading to solvent evaporation. In production of microparticles by spray pyrolysis, each droplet is typically converted into a single product particle. However, for production of nanoparticles (<100 nm diameter), this requires either impractically small droplets or impractically low precursor concentration. Thus, it is preferable to produce many nanoparticles from each precursor droplet. In the examples shown below, we do this by fully evaporating the precursor droplets. Product nanoparticles then nucleate from the vapor phase. A scanning mobility particle spectrometer (SMPS) is used to monitor the size distribution of particles leaving the reactor, which allows us to clearly follow the droplet evaporation and particle nucleation processes as we change reactor conditions, such as temperature or residence time. An alternative means of producing many particles per droplet is to use solution-phase chemistry in droplets of a very non-volatile solvent. In that case, it may be possible to control particle size, shape, etc. as in the solution phase methods described above. Each droplet then serves as a femtoliter-scale reactor that can be heated and cooled, to initiate and quench reactions, on a time scale several orders of magnitude faster than a conventional, macroscopic reactor (3-necked flask). Images: |
|
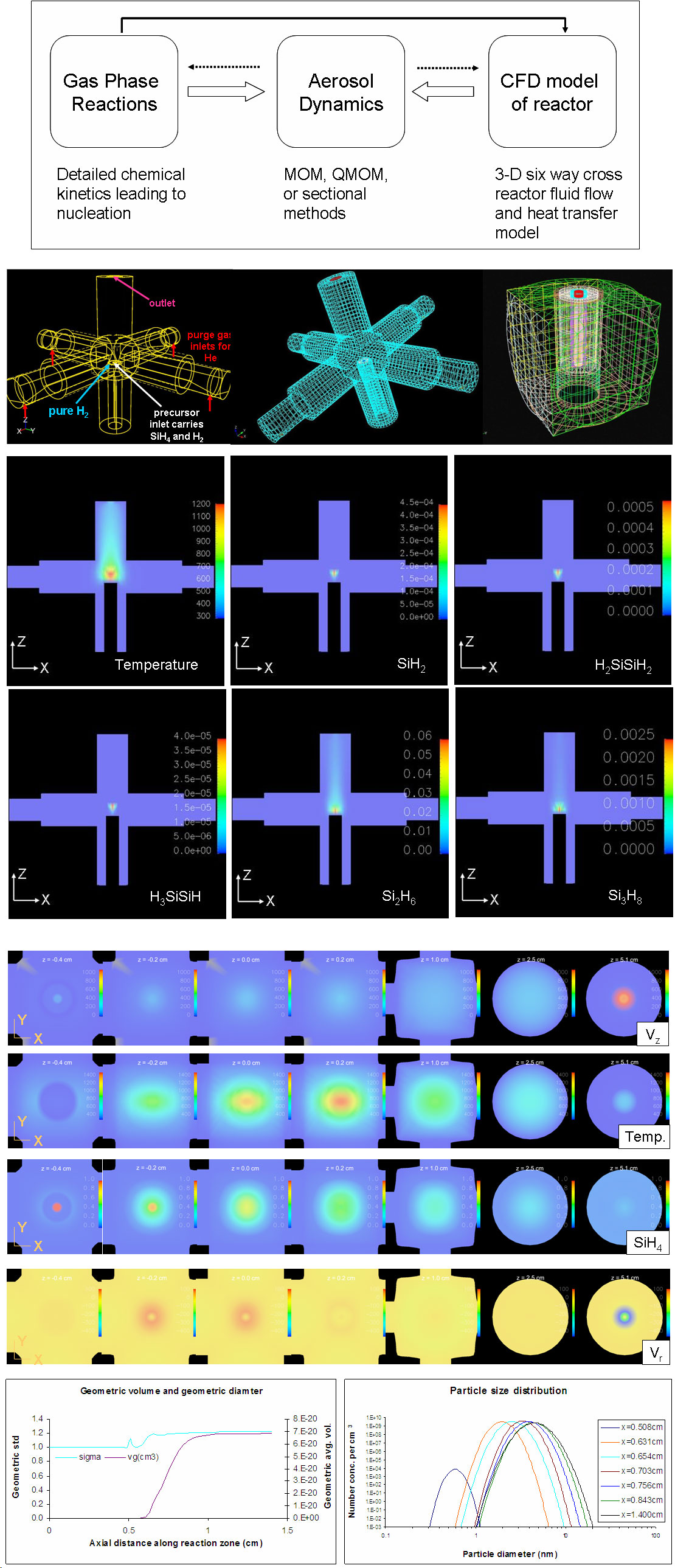
Computational fluid dynamics and aerosol dynamics modeling of nanoparticle formationBrief Introduction:Gas phase (aerosol) processes for producing nanoparticles like the laser-driven and spray pyrolysis processes described above and flame processes, plasma processes, and other techniques being developed and applied by others, often involve very short residence times, very rapid heating and cooling, and complex chemical reactions that convert small precursor molecules into particle nuclei, then lead to growth of those nuclei. This makes fundamental physicochemical modeling of such processes quite challenging. It often also makes experimental measurement of processes occuring during particle formation impossible or impractical. As a result, most processes have been developed using a "black-box" approach in which input parameters (temperature, flows, concentrations) are varied, and the effect on the product powder is observed, without making any fundamental connection between the two. The need for such trial and error experimentation could be dramatically reduced if physicochemically-based predictive models were available. We are working toward the long-term goal of producing such models. Currently, it is not practical to simultaneously treat three-dimensional fluid flow, heat and mass transfer, detailed gas phase and gas-surface reaction kinetics, and detailed aerosol dynamics. Thus, we must make simplifications in one or more aspects of the problem. In the work illustrated below, we have used the MPSalsa reacting flow code from Sandia National Laboratories to model fluid flow, heat and mass transfer, and a few chemical reactions in the detailed three-dimensional geometry of our laser-driven aerosol reactor. One- or two-dimensional temperature and velocity profiles extracted from those simulations, in the region where particle formation occurs, are then used with detailed models of the chemical kinetics and aerosol dynamics to predict particle nucleation, growth, and coagulation, finally producing a predicted particle concentration and size distribution. Images: |
|
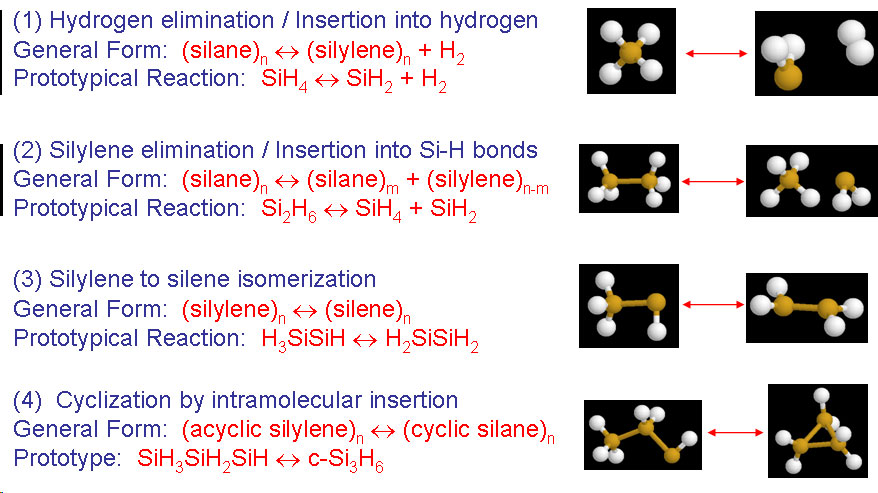
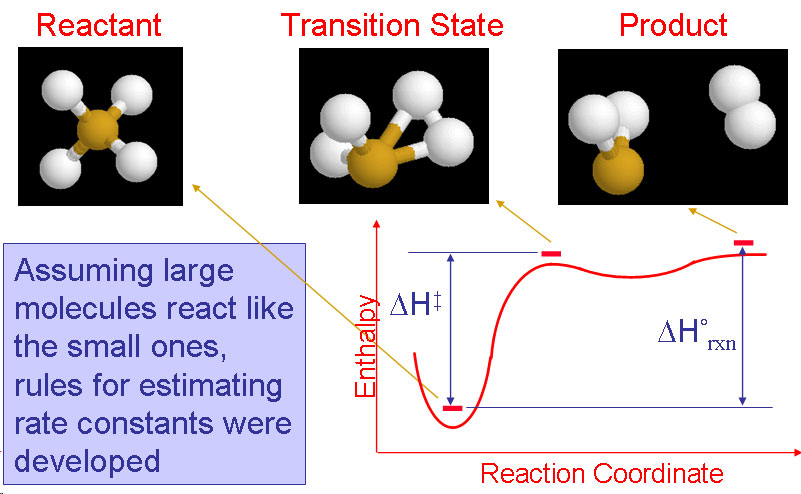
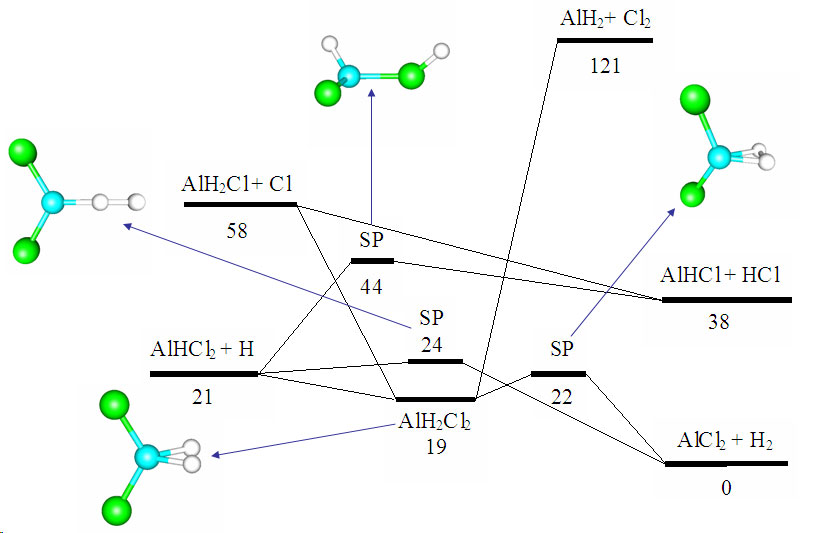
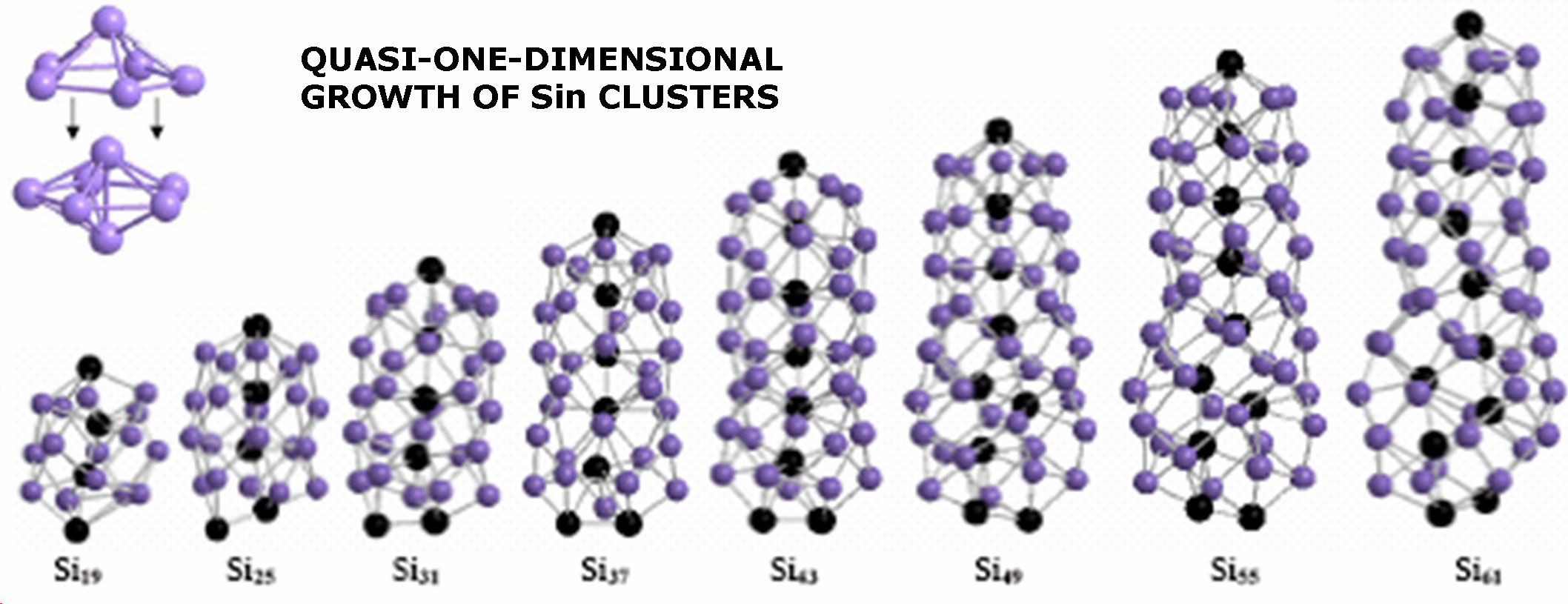
Development of reaction mechanisms using computational quantum chemistryBrief Introduction:Detailed chemical kinetic modeling has been very successful in making quantitative predictions about some systems, particularly the combustion of small hydrocarbons and certain areas of atmospheric chemistry. These systems involve small molecules made up of light elements that are amenable to treatment by computational quantum chemistry, and for which there is a database of experimentally measured elementary rate parameters. The gas phase surface chemistry of many processes in high-temperature inorganic systems, from materials synthesis to propulsion to waste incineration, could in principle be modeled with equal or greater success using detailed chemical kinetic modeling. However, in these systems, there are few experimentally measured rate parameters and even thermochemical properties (enthalpy of formation, etc.) may not be available. While experiments are still the most reliable source for most of this data, they are usually prohibitively expensive and time-consuming. Therefore, we turn to computational quantum chemistry to attempt to build useful detailed chemical kinetic models of these gas-phase processes. This allows us to predict the structure, relative energy, vibrational frequencies ets. not only for reactants and products, but also for transition states and other structures. Using this information in reaction rate theories allows us to estimate rate parameters of chemical reactions. In systems such as particle nucleation, there may be an intractably large number of possible species and reactions. In such cases, we attempt to generalize the predictions for a subset of the possible species and reactions to produce generalized rules for predicting thermochemistry and reaction kinetics. We can then apply these rules algorithmically and use the computer to generate the reaction mechanism as well as to predict the rate parameters and solve the resulting model. Recently, we have been exploring the use of tight-binding molecular dynamics for modeling larger clusters (molecules) than can be handled by ab initio or density functional methods. Images: |
|