Lund Group Projects
Projects in the Lund Group involve Catalysis, Kinetic Modeling, Reaction Engineering and Engineering Education. At the present time (Spring 2019) we have active projects focused on chemical oxygen generators, membrane reactors and problem-solving pedagogy. On the Publications page you will find some information about these projects as well as past projects that involved humin formation during carbohydrate processing, water-gas shift catalysis, nitrous oxide decomposition catalysis, toluene chlorination catalysis, catalytic carbon filament growth, catalytic carbon gasification and other topics. On the People page, you can see the Ph. D. dissertations and M. S. theses from these projects.
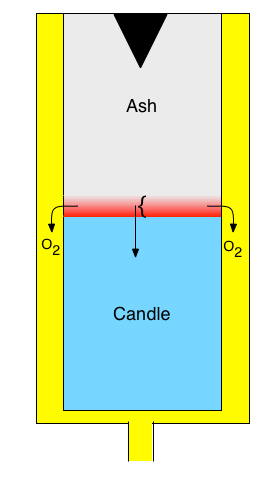 Chemical oxygen generators (COGs) are used in
commercial aircraft and other situations to supply breathable oxygen in
emergencies. They are sometimes referred to as oxygen candles. COGs are small,
cylindrical devices, as represented schematically on the left. The candle
consists of a mixture of a material like NaClO3, binder, a catalyst
and a fuel (for example Sn). Prior to use, there is no ash, and the candle
extends to the top of the device. The black triangle at the top initially
contains an ignition charge; the ignition charge can be similar to the main
candle, but with different proportions of chlorate, catalyst and fuel. To
start oxygen generation, a percussion cap is triggered initiating reactions
(1) and (2) within the ignition charge. The heat released by the reactions is
conducted down into the candle. (The purpose of the fuel is to generate
additional heat, even though it consumes some of the oxygen that is
generated.) When the temperature at a particular point reaches a sufficient
level, the reactions begin to occur at that point. Eventually a slow-moving
front forms, indicated in red in the schematic. Within the front, reactions
(1) and (2) are taking place. Above the front, all the reactants have been
consumed, so no reaction is taking place, and below the front, the temperature
is not high enough for the reactions to occur. Within the front, oxygen is
generated, and it moves to an annular channel and out of the device, providing
breathable oxygen. The front propagates slowly along the length of the device,
continually delivering oxygen until the front arrives at the bottom of the
candle.
Chemical oxygen generators (COGs) are used in
commercial aircraft and other situations to supply breathable oxygen in
emergencies. They are sometimes referred to as oxygen candles. COGs are small,
cylindrical devices, as represented schematically on the left. The candle
consists of a mixture of a material like NaClO3, binder, a catalyst
and a fuel (for example Sn). Prior to use, there is no ash, and the candle
extends to the top of the device. The black triangle at the top initially
contains an ignition charge; the ignition charge can be similar to the main
candle, but with different proportions of chlorate, catalyst and fuel. To
start oxygen generation, a percussion cap is triggered initiating reactions
(1) and (2) within the ignition charge. The heat released by the reactions is
conducted down into the candle. (The purpose of the fuel is to generate
additional heat, even though it consumes some of the oxygen that is
generated.) When the temperature at a particular point reaches a sufficient
level, the reactions begin to occur at that point. Eventually a slow-moving
front forms, indicated in red in the schematic. Within the front, reactions
(1) and (2) are taking place. Above the front, all the reactants have been
consumed, so no reaction is taking place, and below the front, the temperature
is not high enough for the reactions to occur. Within the front, oxygen is
generated, and it moves to an annular channel and out of the device, providing
breathable oxygen. The front propagates slowly along the length of the device,
continually delivering oxygen until the front arrives at the bottom of the
candle.
2 NaClO3 → 2 NaCl + 3 O2 (1)
Sn + O2 → SnO2 (2)
Some of the goals of our COG project are to contribute to a better understanding of the catalytic reaction mechanism, to develop an accurate two-dimensional COG model, to investigate the effects of ignition charge and procedure upon subsequent COG performance and to identify modifications that will improve COG performance. Our approach incorporates integrated experimental studies, kinetic modeling and device modeling wherein each type of activity informs the others.
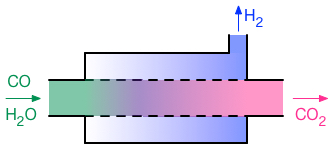 Membrane reactors are combined
reaction-separation devices. A concentric tube membrane reactor is depicted
schematically on the right, in this case for the water-gas shift, reaction
(3). The water-gas shift reaction is reversible, and due to thermodynamic
equilibrium, it does not go to completion at typical operating temperatures.
Some membrane materials, such as palladium metal, are highly permselective for
hydrogen. That is, hydrogen passes through them very much faster than other
gases. In the configuration shown here, the inner tube (represented by a
dashed line) is constructed from that type of membrane. Thus, as hydrogen is
formed within the tube via the water-gas shift, it can permeate through the
tube and into the annular channel. The other gases remain within the tube, and
as a consequence, the concentration of hydrogen within the tube is much lower
than it otherwise would be. This removal of the product hydrogen from within
the tube allows the water-gas shift reaction to proceed beyond the point where
it otherwise would have stopped due to reaching thermodynamic equilibrium.
“Equilibrium shifting” of this type is one very common potential
application for membrane reactors. One membrane reactor project in the Lund
group is focused on the water-gas shift reaction, while the dry reforming of
methane, reaction (4), is being considered in a second membrane reactor
project.
Membrane reactors are combined
reaction-separation devices. A concentric tube membrane reactor is depicted
schematically on the right, in this case for the water-gas shift, reaction
(3). The water-gas shift reaction is reversible, and due to thermodynamic
equilibrium, it does not go to completion at typical operating temperatures.
Some membrane materials, such as palladium metal, are highly permselective for
hydrogen. That is, hydrogen passes through them very much faster than other
gases. In the configuration shown here, the inner tube (represented by a
dashed line) is constructed from that type of membrane. Thus, as hydrogen is
formed within the tube via the water-gas shift, it can permeate through the
tube and into the annular channel. The other gases remain within the tube, and
as a consequence, the concentration of hydrogen within the tube is much lower
than it otherwise would be. This removal of the product hydrogen from within
the tube allows the water-gas shift reaction to proceed beyond the point where
it otherwise would have stopped due to reaching thermodynamic equilibrium.
“Equilibrium shifting” of this type is one very common potential
application for membrane reactors. One membrane reactor project in the Lund
group is focused on the water-gas shift reaction, while the dry reforming of
methane, reaction (4), is being considered in a second membrane reactor
project.
CO + H2O → CO2 + H2 (3)
CH4 + CO2 → 2 CO + 2 H2 (4)
Developing highly accurate, experimentally validated membrane reactor models for water-gas shift and dry reforming membrane reactors is our first goal. Further goals include optimization of hybrid reactor networks that include both conventional and membrane reactors, exploring the parameter space of our models to identify regions where different phenomena (e. g. membrane permeation, reaction kinetics, concentration polarization, etc.) dominate performance, and using our models to pair new membrane materials with appropriate catalysts leading to better hybrid or pure-membrane reactor processes.
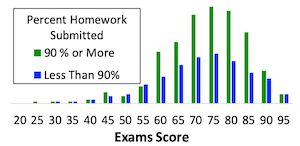 Developing students' problem-solving
mastery is a prominent learning outcome in many engineering courses.
Teachers often assign homework problems as a means of building their
students' problem-solving abilities. However, it is not uncommon to find
that homework fails to meet that objective. For example, the figure to the
left compares the course score distribution of students who completed 90% or
more of assigned homework over a period of 19 years in one particular
problem-solving course to the distribution for those who did less than 90% of
the homework. One would hope and expect that a student who did 90% of the
homework would benefit in some way, but statistically, at the 99% confidence
level, there is no difference between the two distributions. This suggests
that the manner in which homework was being used in that course was not
effective for building the students' problem-solving skills.
Developing students' problem-solving
mastery is a prominent learning outcome in many engineering courses.
Teachers often assign homework problems as a means of building their
students' problem-solving abilities. However, it is not uncommon to find
that homework fails to meet that objective. For example, the figure to the
left compares the course score distribution of students who completed 90% or
more of assigned homework over a period of 19 years in one particular
problem-solving course to the distribution for those who did less than 90% of
the homework. One would hope and expect that a student who did 90% of the
homework would benefit in some way, but statistically, at the 99% confidence
level, there is no difference between the two distributions. This suggests
that the manner in which homework was being used in that course was not
effective for building the students' problem-solving skills.
The present focus of our research on problem-solving pedagogy is examining the effects of explicit teaching of problem-type identification, explicit enumeration of generalized solution procedures for specific problem types, scaffolded, in-class practice in problem-type identification and application of generalized solution procedures and initial grading of homework on the basis of effort with required homework wrappers. The goal of our work is to identify teaching strategies that cause students to focus on the solution process, and not the just the answer to the problem, when they are engaged in a problem-solving activity.
 Educational tools for teaching kinetics and
reaction engineering in a “flipped” classroom are being developed
in another Lund Group project. Dr. Lund has developed a set of tools for both
students and teachers to use in a kinetics and reaction engineering course
being taught as a flipped class. Those materials are available on the AFCoKaRE
(A First Course on Kinetics and Reaction Engineering) website (click on the
image at the right to visit that site). The current project involves
developing and testing in-class tools that can be used to foster the
metacognitive development of the students. The tools are a combination of
purpose-designed, in-class worksheets, homework assignments and homework
wrappers. These tools will be added to the AFCoKaRE website once development
and assessment are complete.
Educational tools for teaching kinetics and
reaction engineering in a “flipped” classroom are being developed
in another Lund Group project. Dr. Lund has developed a set of tools for both
students and teachers to use in a kinetics and reaction engineering course
being taught as a flipped class. Those materials are available on the AFCoKaRE
(A First Course on Kinetics and Reaction Engineering) website (click on the
image at the right to visit that site). The current project involves
developing and testing in-class tools that can be used to foster the
metacognitive development of the students. The tools are a combination of
purpose-designed, in-class worksheets, homework assignments and homework
wrappers. These tools will be added to the AFCoKaRE website once development
and assessment are complete.


