This page shows different molecular geometries as described by the Valence-Shell-Electron-Pair Repulsion Model. This model takes into account the repulsion effects of electron pairs. The geometry is determined by both bonded atoms and electron pairs. The total number of atoms bonded and electron pairs around the central atom is referred to as the number of positions. On this page, the blue atoms are the central atoms, and the red are the bonded atoms. Also, when an atom is "deleted" it can be thought of as an electron pair. Some of the examples given are hypothetical.
Click on the following images to go to the corresponding structures.
|
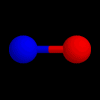
This is the structure of molecules with 1 position. These have 1 atom and 0 electron pairs, such as Hydrogen, H2.
|
|
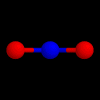
These are the structures of molecules with 2 positions.
|
|
|
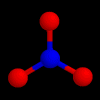
These are the structures of molecules with 3 positions.
|
|
|
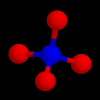
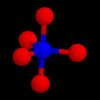
These are the structures of molecules with 4 positions.
|
|
|
These are the structures of molecules with 5 positions.
|
|
|
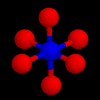
These are the structures of molecules with 6 positions.
|
select not (center atom) color atom (color)See the explanation of disappearing atoms given for the crystal structures.