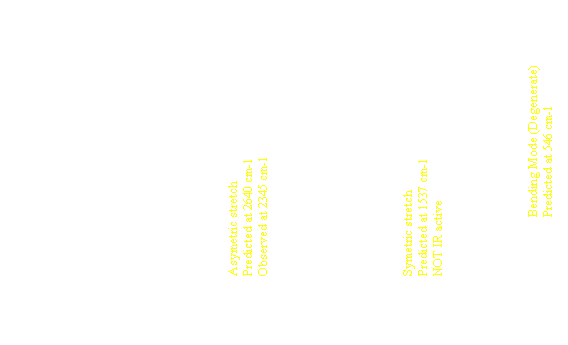
| Energy can be stored in molecules as translational, rotational and vibrational energy. Translation can occur in the x, y or z direction. Rotation can occur around the x, y or z axis, except for linear molecules which only have two axes or rotation. Vibrations involve movements of the atoms of a molecule which produce no net translation or rotation. These various movements are a result of the combination of the normal modes of vibration. For a triatomic molecule these normal modes are symmetric, asymmetric and bending vibrations. In symmetric vibration, the two bonds shorten and lengthen together. In asymmetric vibration, one bond shortens while the other lengthens. In bending vibration, it is the bond angle that oscillates. |