Dendritic Polymers and Their Potential Applications
Abstract
Dendritic polymers are belonging to a special class of macromolecules. They are called "Dendrimers." Similar to linear polymers, they composed of a large number of monomer units that were chemically linked together. Due to their unique physical and chemical properties, dendrimers have wide ranges of potential applications. These include adhesives and coatings, chemical sensors, medical diagnostics, drug-delivery systems, high-performance polymers, catalysts, building blocks of supermolecules, separation agents and many more.
1. Introduction
1.1. The Origin of Dendrimers
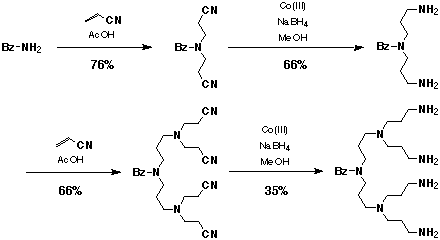
The name dendrimer is derived from Greek words dendron meaning "tree" and meros meaning "part." A major difference between linear polymers and dendrimers is that a linear polymer consists of long chains of molecules, like coils, crisscrossing each other. A dendrimer consists of molecular chains that branched out from a common center, and there is no entanglement between each dendrimer molecules. The first synthesis of these macromolecules is credited to Fritz Vogtle and coworkers in 19781. It consisted of Michael Addition of acrylonitrile to primary amine groups. Each successive step involved reductions of the nitrile groups followed by additions of acrylonitrile (Figure 1).

Figure 1: "Cascade" synthesis of poly(propylenimine) by Vogtle (ref 1).
The synthesis of dendrimers is very difficult and expensive. The industry sector was not keen to invest in dendrimer research. Until recently, key-pioneering works by Tomalia and Frechet solved many of the difficulties. Dendrimer research is still at its infant stage.
1.2. The Structures of a Dendrimer Molecule
Dendrimers have a globular configuration with monomer units branching out from a center cord (Figure 2, ref 22). The structure is highly defined and organized. The number
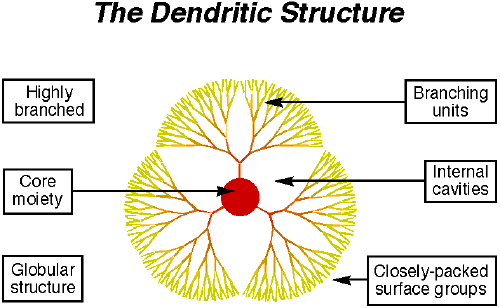
of branches increases exponentially extending from core to the periphery. The branching would come to a stop when the steric hindrance stopped any further growth. There are three distinct architectural components2 (Figure 3). The multi-functionalized core (initiator core) forms the heart of the molecules; all branches emanate from this core.
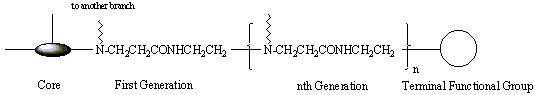
Figure 3: Components of a dendrimer, poly (amidoamine)
The monomers that attach to the core form the first branches (Tomalia called them the First Generation). On the successive generations, two monomers will attach to the ends of the monomers in the previous generation. At the terminating generation, a terminal functional group is added to the tail of the monomer. Most of the chemical properties of the molecule depend on types of terminal groups. The physical properties of the molecules, such as solubility and viscosity are also affected by the terminal groups.
Some of these dendrimers have diameters that are greater than ten nanometers3. The molecular weights range from about 50,000 to 200,000 g/mol. The outer surface area of the molecule increases with the number of generations. There is a significant of void space within the molecule. These voids consist of channels and cavities4. These unique geometries give the molecule special properties such as adhesiveness and ability to entrap foreign molecules. The calculations of the molecular weight and other useful quantities about the dendrimer molecules are presented in a paper by Tomalia2. The number of terminal groups is easily calculated as follows:
Number of terminal groups = Nc (Nr)G
Where Nc is the number of branches at the core (core multiplicity); Nr is the number of branches on each monomer unit (repeating unit multiplicity); G is the number of generation. The degree of polymerization can be computed using these quantities.
![]()
![]()
Mw=
where Mc, Mr, and Mt are the molecular weight of the core, the repeating monomer, and the terminal group respectively.
1.3 Properties of Dendrimers
Theoretically, dendrimers are monodispersive. All molecules have the exact same molecular weight and structure. Due to minor defect during the synthesizing process, the polydispersity index is about 1.001. Polydispersity of 1.0007 for PAMAM has been reported5. The intrinsic viscosity of dendrimers has a peculiar behavior. It increases with increasing molecular weight (number of generations). Contrary to linear polymers, the viscosity will reach a maximum value then starts to decline6 (Figure 4). It is suggested
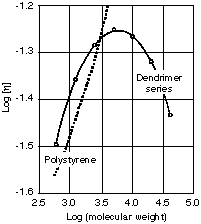
Figure 4:
comparison between the intrinsic viscositiesof polystyrene and dendritic polyether (ref 6).
that the space between the branches is smaller in higher generation dendrimers than lower generation dendrimers. The decline in viscosity is a consequence of prohibiting the interaction of the outer branches between molecules at a higher generation6. The glass transition temperature (Tg) of dendrimers follows similar trend. It reaches to a maximum Tg and levels off at higher molecular weights6,7. This behavior is explained by the absence of entanglement at higher molecular weights6.
2. Synthesizing and Designing Dendrimers
2.1. Divergent Growth Method
This type of synthesis involves two steps: The activation of the functional surface groups and the addition of branching monomer units8. The reaction starts at the core.
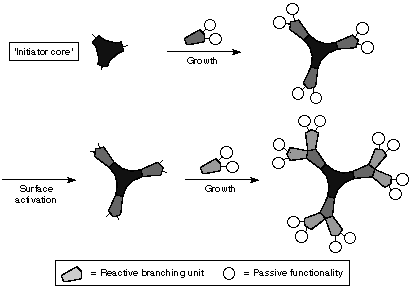
Figure 5. Divergent growth method (ref 22)
The initiator core contains several reaction sites. The first generation monomer units react with the core readily. Once all reactive sites are taken, the addition stops. Since the end groups on the first generation are protected, addition of monomers to the end chain is impossible. The end groups must be activated before any further addition. The passive functionalities on the end groups are removed by a secondary reaction. Additional monomers are attached to the molecule. Steps are repeated for synthesizing higher generations. Ideally, one can control the structure and molecular weight of the molecule precisely. Divergent growth method is labor intensive and repetitive. In successive generation growth, side reactions and incomplete additions become more apparent9. This is attribute to steric hindrance. In each step, the desire products must be purified. The overall yield is considerably small. One of the great advantages of this method is the ability to modify the surface of the dendrimer molecule. By changing the end groups at the outermost generation, the overall chemical and physical properties of the dendrimer can be configured to specific needs10. It is due to this versatility that sparked the interest in dendrimer research. Tomaliaís PAMAM dendrimers were synthesized using divergent method, starting with an initiator core and expanding to the periphery2,9.
2.1. Convergent Growth Method
One of the shortcomings of divergent growth method is that the outermost generation has only one kind of functional group. Convergent growth method would eliminate such weakness. This method was first introduced by Frechet11. The reaction starts at the periphery and proceeds to the core. Similar to divergent growth, it involves two steps:
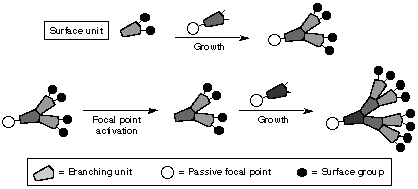
Figure 6: Convergent growth (ref 22)
the attachment of the outermost functional groups to an inner generation and the attachment of the inner generations to the core. The structural units before the final attachment to the core is called the "wedge." Usually, three to four wedges attach to the core. Each wedge can have different functional groups at the periphery. Thus, the
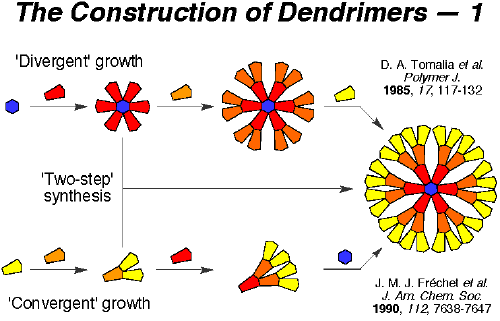
Figure 7: Comparison between divergent and convergent growth.
making of unsymmetrical dendrimers is possible. This modification is useful in monolayer formation at the organic-aqueous interface11. Half of the dendrimer is submerged in the water phase, while the other half is in the organic phase.
A combination of these two methods can be use to suit for special needs.
3. Potential Applications of Dendrimers
3.1. Medicinal Applications
The idea of dendrimer serving as host for foreign molecules was first stumble upon by Meijer12. He trapped several small molecules (Bengal Rose dye) in the cavities of water soluble dendrimer molecules with a diameter about 5 nm. The "dendritic box" is a fifth generation poly (propylene imine) consisting of 64 functional groups at the periphery. The trapped foreign molecules cannot diffuse out of the box. Only upon prolong heating, the trapped molecules were able to escape. It is possible to use the "dendritic box" as vehicle for drug delivery. Meijerís group has been designing a box that could be opened enzymatically or photochemically. This unique feature is also explored by Tomaliaís group13. The capability of hosting small organic molecules in water is the key to transport biological molecules.
Frechetís group at Cornell is working on a dendrimer for chemotherapy14. A chemotherapeutic drug is weakly bonded to the periphery of a dendrimer. Other functional groups add to the dendrimer to increase water solubility. Once the dendrimer reaches its target, the bond between the drug and the dendrimer is cleaved (enzymatically or photochemically). Since dendrimers are inert and stable, they are nontoxic to human. It was shown that dendrimers could eliminate through the kidneys as urine14.
Willich, a German scientist is working on a dendrimer for magnetic resonance angiography and is currently entering clinical trials15. The dendrimer is polylysine with gadolinium ion complexes on the end groups. Dendrimer provides multiple bonding sites on the periphery, allowing many MRI contrasting agent complexes to attach to one dendrimer. One dendrimer molecule can host up to twenty-four contrasting agent complexes and hence attaining higher signal-to-noise ratio15. The dendrimer prevents any complex from diffusing into untargeted area. Thus, a better contrast MRI picture is obtained.
Several types of PAMAM dendrimers have been tested as gene vectors for gene therapy15. Experiments showed that PAMAM dendrimers are effective transfection agents, providing high successful rate of transferring genetic materials into the cell. Dendrimers also tested in boron neutron capture therapy15. It is possible to attach boron complexes and antibodies on the surface of the dendrimer. While the antibodies target at the cancer cells, the boron complexes capture neutrons from an external source and release energetic radiation that can kill the cancer cells.
3.2. Dendrimer Films
Frechetís group at Cornell is working on polyether dendrimers films that can isolate metal ions3. His target application is for signal amplification in fiber-optic communication technology. Apparently, his dendrimer coatings on the metal ions are able to prevent interference between ions when they excited by light. Crooks and Well at A&M are exploring the possibilities of using dendrimer films as sensitive interfaces for sensing applications16. The dendrimers formed a thin monolayer film onto a gold surface. When it exposed to volatile organic compounds, the film was able to capture the volatile molecules. The contains of the film were then analyzed by a device called surface acoustic wave mass balance. The functionalities on the periphery of the dendrimer molecule could be modified to sense different organic compounds selectivly16. Dendrimer films also serve as anti-corrosive coating on metal surfaces. The film is able to trap corrosive agent in the dendritic cavities, preventing any diffusion to the surface of the metal16.
3.3. Interphases Applications
As previously discussed, the convergent method is able to make unsymmetrical dendrimers. This arrangement allows the dendrimers to form monolayers at the gas-liquid interfaces or aqueous-organic interfaces17. Amphiphilic dendrimer are useful in forming interfacial liquid membranes for stabilizing aqueous-organic emulsion (Figure 8). One
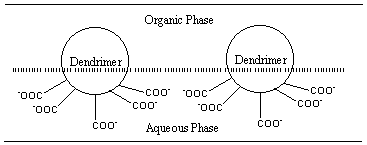
Figure 8:
Liquid membrane of amphiphilic dendrimer at the interface between water andan immiscible organic solvent (ref 17).
other application is to use dendrimer film to extract chemical compounds between two phases. Dendrimers with carboxylate chain ends can form micelles in water. Their hydrophobic interiors dissolve organic molecules that are insoluble in water. They act like carrier for organic molecules in aqueous phase. This arrangement holds promise for the development of organic chemistry in aqueous medium17. Hydrophilic dendrimers with hydrophobic functionalities on the periphery form micelles in organic solvents. These types of dendrimers can extract organic compound from the water phase to the organic phase5. Dendrimers films also can use as purifiers. They can selectively permit molecules to diffuse through the interface17.
3.5. Catalysis and Reaction Sites
Catalysis is one of the most promising applications in dendrimer research. Dendrimers have nanoscopic cavities that act like microenvironment for molecular reactions5. The cavities provide nanoscale reactor sites for catalysis. There are two possible catalytic sites being investigated, one at the core and the other at the surface respectively. Many attempts have been made on using dendrimers to enhance reaction rate and reaction selectivity. There is a micropolarity around the core, thereby influencing its molecular recognition and catalytic properties14. One of the possible schemes for catalytic reaction in water is shown in figure 8. It is found that dendrimers are very useful in enantiomeric
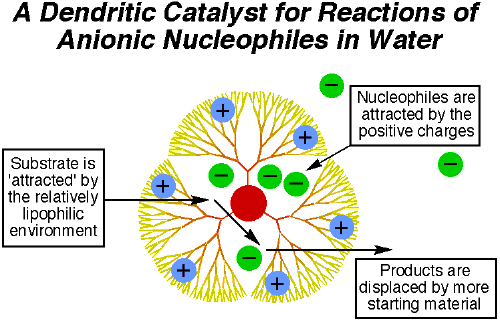
Figure 9:
diethylzinc addition to benzaldehyde18.
around the catalytic core and inducing regio and shape selectivity19. The first peripheral catalytic site has been reported by Ford et al5,20. Since there are multiple of catalytic sites concentrated at periphery and the dendrimer provides a good anchorage, the catalytic activity increase significantly comparing to one-dimensional catalytic site. Detty et al has been working on a dendrimer catalytic film that can kill algae and bacteria in sea water21.
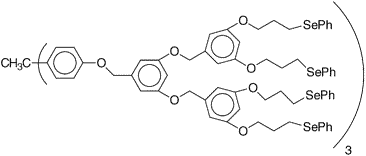
Figure 10: The selenides groups at the periphery catalyze peroxide activation of bromine
Cation21
Phenylseleno groups are connected to a third generation dendrimeric polyether molecule. The selenides catalyze the oxidation of bromide ion with hydrogen peroxide to give positive bromine radicals21. These radicals are very toxic to algae and bacteria. Its potential application is in ship building industry. The dendrimer film on hulk will provide protection against the accumulation of algae.
3.6 Other Applications
Frechetís group is investigating a dendrimer that can harvest light14. The harvested light energy can transform into chemical energy for reactions, electric current or convert the energy into monochromic light. A laser dye has been placed at the dendritic core. The system acts like an optical amplifier14. One other potential application is to use the dendrimer as a host for polymerization22. It is possible for polymerization to occur in the
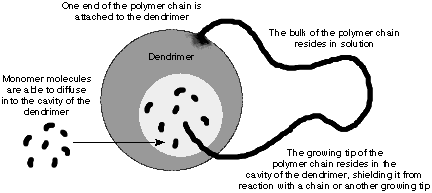
Figure 11:
Scheme for control polymerization (ref 22).cavities of the dendrimer and is well protected, therefore avoiding termination with other polymerization chains.
4. Conclusion
The designing aspects of dendrimers can be control carefully. One can synthesize dendrimer with certain molecular mass and structural conformation. The unique physical and chemical properties of dendrimers have demonstrated great versatilities in variety of applications. The dendrimer topologies provide many special properties such as in interphase applications and in nanoscale reactors. Dendrimers can be functionalized at the periphery or at the core with variety of functional groups for catalysis actions. Dendrimers have successfully been used in medicinal applications such as diagnostic tools and eventually in drug delivery.
5. Reference:
(http://pubs.acs.org/hotartcl/cenear/960603/dend.html)