KaptonÒ Wiring Report
Tom Hanselman
Dan Boek
Jason Womer
KaptonÒ is a registered trademark
of E.I. du Pont de Nemours and Company
Swiss Air Flight 111
Electrical
Problems may have sparked the Swiss Air Flight 111 crash. The plane crashed off
the Nova Scotia coast on September 2nd, killing all 229 people
aboard. The exact cause of the crash is unknown, however, evidence points
towards a rapid and deadly electrical fire. Pilot Urs Zimmermann noticed smoke
at approximately 10,000m and 16 minutes later the plane slammed into the
Atlantic Ocean off Peggys Cove, N.S. Canada. Swiss Air Flight 111 had
approximately 240 km of wires running throughout its belly to bring passengers
a premium video and gambling system. Most of the wires used KaptonÒ as the primary material.
Swissair is a pioneer in installing such devices in the intensely competitive
market for business and first class flyers. Seats aboard Swiss Air Flight 111
had a video screen that pops out of the armrest like a tray table. Passengers
can use this screen to play video games, music, or gamble on international no
U.S. flights. It was this luxury which could have lead to the crash. The web of
wires increases the chance that one of the wires will crack due to vibrations or
moisture in the plane. The increase in wires also generates more heat and could
have lead to the problem. Salvage crews found evidence of heat damage on the
ceiling of the cockpit and first-class cabin, which is where the heart of the
in-flight-entertainment system is located. In the future this type of video
system should be connected to a separate area of the plane where vital networks
in the plane will not be affected thus buying time for an emergency landing.
The new system of wires should be located in an area where pressurized bottles
of Halon are ready to douse the fire if a problem arises.
Fire Prevention
Often
people die on planes due to smoke inhalation. Improving methods in fire
prevention could be accomplished in many ways. PHA is a plastic, which emits
water vapor when it burns. It brakes down into a flame-resistant compound and
could be used in many fire prevention applications. The Federal Aviation
Administration or FAA announced in October of 1998 that Mylar insulation used
in nearly 12,000 passenger jets must be replaced to reduce the chance of fire.
Mylar insulation easily catches on fire and creates a cloud of smoke. It is
believed that this type of insulation was set ablaze by a short circuit in the
electrical equipment aboard the Swiss Air Flight 111. Adding flame retardant
materials to planes could help. Chlorine and Fluorine when added to materials
help increase that materials fire resistance. The trade off is that once this
type of material eventually catches on fire the smoke it emits is toxic.
The investigation into Swiss
Air Flight 111 revealed safety deficiencies in crew training and awareness, and
procedures related to in-flight firefighting. The TBS safety board issued the
following recommendations to address safety deficiencies in firefighting
training in pilots and crew.
1)
A
lack of a coordinated and comprehensive approach to in-flight firefighting.
2)
Smoke/fire
detection and suppression systems are insufficient.
a)
There
are no smoke/fire detection and suppression systems in the cockpit or cabin or
any area not considered a fire zone.
3)
The
importance of making prompt preparations for a possible emergency landing is
currently not recognized.
a)
This
is due to company policy and the feeling that it is an inconvenience.
4)
Access
to critical areas within the aircraft is inadequate.
a) There has been littler or
no training provided to aircraft crew on how to access areas behind electrical
or other panels.
Crash Statistics
Where would you feel the safest sitting on an aircraft? The safest part of an aircraft to sit if your worried about the plane breaking up is towards the front of the wings near the engines because this is the strongest part of the aircraft. The fuel tank is located under this section of the plane and the plane is structurally the strongest here. The trade off with sitting here is that it is the noisiest part of the aircraft.
In general the larger the vehicle the safer the travel so no one should be worried about flying. As vehicles increase in size there are more safety regulations placed upon them so as expected the travel becomes safer. Some interesting safety facts about flying include:
1) You are more likely to
die by being kicked to death by a donkey than in a plane crash
2) You are more likely to be
crushed by a falling object
3) You are much more likely
to be killed by your spouse
In
fact you have one chance in about 7 million from dying in a plane crash.
There
are ways to increase you chances if you are not satisfied with the 1 in 7
million statistic. I will call these handy hints for the nervous traveler.
1)
70%
of incidences in recent years have occurred during take-off or landing so when
booking your travel planes try and book non-stop flights.
2)
Know
you surroundings because 71% of the people who die in crashes die after the
plane comes to a complete stop. Take note of where the nearest emergency exit
is.
3)
Choose
a large aircraft because as stated earlier the larger the vehicle the better. I
would recommend a plane with the ability to seat over 80 passengers.
Improving Insulation
In
studying the effect of rubber on the Environmental Stress-Crack Resistance or
(ESCR) the choice of a base resin is important. Depending on the resin and
rubber combination you choose the (ESCR) can double or increase by 50-fold.
KaptonÒ could be improved with
slight variations in its production and perhaps different types of resins
should be looked at. The higher the molecular weight of polyisobutylenes the
more effective its performance as a stress crack additive.
Degradation
by radiation-induced current in crystalline materials such as polyethylene is
affected more by the degree of crystallinity than its perfection.
Since
1993 KaptonÒ has been improved by
wrapping it in a tough Teflon coating. Other ways to improve KaptonÒ would be to try other
copolymers in the production of KaptonÒ. Copolymers usually
increase a materials ability to withstand an impact and vibrations can lead to
cracks in Kapton wiring. Teflon may not be the best choice although it is a
good choice. In an article I found from 1998 FAA officials said the old forms
of KaptonÒ might be removed from
hundreds of planes.
Properties
of Kapton®
Kapton® is a registered
trademark of E.I. du Pont de Nemours and Company.
Kapton® and other polymers are good insulators
because the carbon-carbon bond with two hydrogen atoms attached keeps electrons
localized. Electrons must become
delocalized and flow in order for a structure to become conductive. The chemical structure of Kapton® can be
seen below.
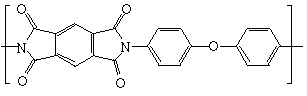
However, chemistry always provides for an exception to its rules. The 2000 Nobel Prize was given to Alan Heeger, Hideki Shirakawa and Alan MacDiarmid “for the discovery and development of electrically conductive polymers.” The research team doped polyacetylene with strong electron acceptors such as iodine. The polymer began to conduct nearly as well as metal, with a conductivity 1010 times higher than pure polyacetylene. Doping caused the polymer to absorb and reflect infrared light, whereas the pure polymer is transparent. Kapton® polyimide film by DuPont is an electrical insulation material with outstanding thermal, mechanical, chemical and electrical properties. Kapton® has been used successfully in field applications where the environmental temperatures were as low as -269°C and as high as 400°C. It offers excellent adhesive bondability and resists high mechanical stress during assembly operations. Kapton® is resistant to most chemicals, solvents, lubricants and fuels.
Uses of Kapton®
Since properties of Kapton® are necessary in many
technological areas the polymer’s use is very diversified.
Some
Kapton® aerospace applications include:
- Motors / alternators
(insulation)
- Wire & Cable
Insulation
- Smoke hoods
- Cockpit sun shade
- Flexible circuitry
- Acoustic insulation
Airbus uses Kapton® as slot liner for the stator in
the alternator, which functions as a back-up to the generators in case of a
major electrical failure. Kapton® is
also used as primary winding insulator of the transformer rectifier in the
Airbus, providing constant DC current to the inboard computers and flight
instruments. Tape created from Kapton®
that can be pressure sensitive is used as insulation material for wires and
cables to separate the layers and insulate the connection wires.
Some Kapton® automotive applications include:
- Diaphragms; air bags,
air-conditioning systems, fuel pressure regulator, etc.
- Flexible circuit for
dashboard
- Gasket
- Radiator plug
- Spark plug boot
- Bar code labels
- Speaker parts; cones,
domes, spiders, surrounds, voice coils
Kapton® is used for diaphragms, insulators, gaskets
and parts that must withstand adverse operating conditions in automotive
applications.
Some Kapton® electrical insulation applications
include:
- Wire and cable
insulation
- Formed coil insulation
of traction motors
- Mica tapes
- Magnet wire insulation
- Transformer and
capacitor insulation
- Transformers
- Downhole pumps
High speed trains with traction motors use Kapton®
in order to withstand the damaging effects of corona. The voltage and magnet wire life are increased and improves
operational reliability, increased power and substantial weight savings.
Some
Kapton® bondable applications include:
- Bar code labels
- Belts
- Fuel sensors
- Heating elements
- Pressure sensitive tape
- Smoke hoods
- Blood bags
Kapton® polyimide film was designed to function as
an excellent adhesive and bonding sheet for high temperature performance
material constructions. Kapton® can be
coated with heat sealable Teflon® fluorocarbon resin and is used in the wire
industry where standard glues and adhesives fail.
Some Kapton®
thermal management applications include:
- Heating elements
- Coil insulation
- Smoke hoods
- Power supplies
- Heater circuits
- Copier belts
- Carrier belts
Kapton® is used as a heat conducting product in
heating elements for a variety of different domestic appliances, including
hot-plates and coffee pots. Kapton®
ensures the optimal heat transfer between the film and the item and with its
electrical insulation effect and dielectric strength, offers a high degree of
functional reliability.
Some Kapton®
electronic applications include:
- Flexible circuits
- Tape automated bonding
- Heat sinks
- Spacers, stiffeners
- Masking tapes
- Spiral tubes
Kapton® can withstand extreme temperatures and has
excellent flexibility. It usually does
not break from flexing and resists shrinkage.
It allows excellent bonding to copper and forming characteristics.
Degradation of Kapton® and other Polymers
Even though Kapton® has outstanding mechanical
properties it is still subject to cracking with lots of vibration. Maintenance workers may bend or rub against
wires or even expose them to chemicals.
Moisture is prevalent in the wings of airplanes due to
condensation. Long polymer chains of
Kapton® break down, and the insulation becomes brittle, developing small cracks
that in turn let in even more moisture.
Arcing is the result of this moisture degradation. The arcs begin to carbonize the insulation
creating an excellent conductor. After
enough carbon has been built up there can be a large explosive flashover with
exposed wires giving of molten metal.
Some experts believe that that thermal degradation
causes problems with Kapton®.
Temperatures in excess of 10,000 degrees Celsius can occur during
arcing. This is a high enough
temperature to degrade Kapton®. The two
classes of thermal degradations are depolymerisation and substituent reactions.
Depolymerisation occurs by the breaking of the main
polymer chain backbone so that at any intermediate stage the products are
similar to the parent material in the sense that the monomer units are still
distinguishable. The dominant product
may be monomer.
In substituent reactions the substituents attached
to the backbone of the polymer molecules are involved so that the chemical
nature of the repeat unit is changed even though the chain structure may remain
intact.
Another form of polymer degradation that may affect
Kapton® in uses other than airplanes is photo-degragation. Sunlight is an important factor in the
deteriorative ageing and weathering process that occur in commercial
polymers. Radiation from the sun that
reaches the earth’s surface extends from the infra-red (> 700nm), through
the visible spectrum (400<700nm) into the ultra-violet (<400nm)
spectrum. The 700, 400 and 300nm
photons have energies of 170, 300 and 390 kJ/mol respectively. The strengths of C-H and C-C bonds are
approximately 340 and 420 kJ/mol respectively in most polymers. The energy of the quanta of the UV and
possibly of the visible components of sunlight is sufficient to break chemical
bonds and that the shorter wavelengths will be the more effective.
Absorption of radiation is an essential first step
to photo-degradation. Strongly absorbed
radiation will be attenuated as it passes through the polymer and reaction will
be concentrated in the surface layers.
Photo-degradation’s first chemical step is usually hemolytic bond scission to form free radicals. In the presence of oxygen, these radicals will react rapidly. Visible and especially ultra-violet radiation are particularly effective initiators of oxidation.
Since Kapton® degradation in airplanes is not
definitely moisture, the cause could be oxidation. Degradation is almost always faster in the presence of
oxygen. The rate of oxidation is
normally slow at first, but then accelerates and finally reaches a constant
rate. Ground state oxygen exists in the
triplet state and is a diradical.
Oxygen normally reacts with organic compounds in a radical chain
reaction involving the ground state.
The two reactions below lead to the formation of hydroperoxides.
R. + O2 → ROO. (1)
ROO.
+ RH → ROOH + R. (2)
Reaction 1 is a radical pairing process and has a
low activation energy that occurs with high frequency. The second reaction involves the breaking of
a carbon-hydrogen bond and has a higher activation energy. The second reaction determines the rate of
oxidation. Several termination
reactions are possible and can be seen below.
2R. ® R-R (3)
R. + ROO. ® ROOR (4)
2ROO.
® ROOR + O2 (5)
Normally reaction 2 is rate determining,
alkylperoxyl radicals are the dominant radical species present in autoxidation
and termination occurs primarily through reaction 5. If oxygen access is limited by diffusion reactions 3 and 4 play
important roles.
The main causes for the degradation of Kapton® are vibration and moisture according to theory. Arcing is the result of mechanical damage. Thermal degradation then takes place after arcing.
Insulation Requirements for wires
The following table contains the requirements for machine-tool wires and cables for use as specified in the National Electrical Code (ANSI/NFPA 70). Tests are performed and thus the requirements are set based on finished wires and cables.
|
Conductora
size |
NEC construction
A (Nylon jacket not used) |
NEC construction
B |
||||||||
|
Minimum average
thickness of the insulation |
Minimum
thickness of any point of the insulation |
Minimum average
thickness of the insulation |
Minimum
thickness of any point of the insulation |
Minimum
thickness of any point of nylon jacket |
||||||
|
Mils |
mm |
Mils |
mm |
Mils |
mm |
Mils |
mm |
Mils |
mm |
|
|
22 - 7; 20 - 12 AWG |
30 |
0.76 |
27 |
0.69 |
15 |
0.38 |
13 |
0.33 |
4 |
0.10 |
|
11 - 10 |
30 |
0.76 |
27 |
0.69 |
20 |
0.51 |
18 |
0.46 |
4 |
0.10 |
|
9, 8 |
45 |
1.14 |
40 |
1.02 |
30 |
0.76 |
27 |
0.69 |
5 |
0.13 |
|
7, 6 |
60 |
1.52 |
54 |
1.37 |
30 |
0.76 |
27 |
0.69 |
5 |
0.13 |
|
5, 4 - 2 |
60 |
1.52 |
54 |
1.37 |
40 |
1.02 |
36 |
0.91 |
6 |
0.15 |
|
1 - 4/0 |
80 |
2.03 |
72 |
1.83 |
50 |
1.27 |
45 |
1.14 |
7 |
0.18 |
|
213 - 500 kcmil |
95 |
2.41 |
86 |
2.18 |
60 |
1.52 |
54 |
1.37 |
8 |
0.20 |
|
501 - 1000 |
110 |
2.79 |
99 |
2.51 |
70 |
1.78 |
63 |
1.60 |
9 |
0.23 |
|
a A conductor is appropriate for use with a
cross-sectional area that does not correspond to one of the AWG or kcmil
sizes in Table when the finished wire or cable complies with the marking
requirement in and with each of the following: |
||||||||||
|
a) The conductor shall have a dc resistance that is not
larger than the resistance determined by interpolation(by cross-sectional area)
between the resistances of the next larger and smaller sizes shown inthe
applicable Table or . |
||||||||||
|
b) The number of strands, the thicknesses of insulation,
the thickness of any nylon jacket, and other particulars shallcomply with the
requirements applicable to the next smaller size shown in Table . |
||||||||||
These requirements are set for wires and cables that
are used at temperatures of 90°C (194°F) and lower in dry locations. These specs
are also for use where the wire or cable is exposed to moisture, oil, coolants,
or any other liquid in temperatures of 60°C (140°F) or lower.
This table includes the specifications for both nylon jacketed and
non-jacketed wires. It is possible to
manufacture such wires that do not comply with these specifications, however
the finished product must go through a series of tests in order to prove the
safety and reliability of the coating.
References
Cited
•Maclean’s
2000 Dec 18 p 25, “Fire hazards.”
•Washington
Monthly 1997 v 29 n 9 p 44, “Fly the fiery skies: long after ValuJet, many
planes still don’t have smoke detectors or fire extinguishers in their cargo
holds.”
•U.S.
News and World Report 1998 March 2 v 124 n 8 p 62, “Unsafe skies over Asia: air
saftey.”
•“Air
Transport Association” URL: http://www.air-transport.org/public/speeches/view1997.asp?UniqueID=38
•“AMIGOINGDOWN.COM”
URL:http://www.amigoingdown.com/
•The
Economist (US) 1997 Jan 11 v 342 n 7999 p 55, “Fasten your safety belts.”
•The
Economist (US) 1997 Jan 11, v 342 n 7999 p 13, “How safe is your airline?”
•Fortune
1995 June 12 v 131 n 11 p 20, “One reason not to get a window seat.”
•People
Weekly 1997 Oct 20 v 48 n 16 p 125, “Crash course: even if your plane goes
down, says an expert, there may be simple things you can do to survive.”
•U.S.
News and World Report 1998 March 2 v 124 n 8 p 62, “Unsafe skies over Asia: air
safety.”
•Time
2000 Nov 13 v 156 I 20 p 91, “ How to Survive a Crash: The Singapore Airlines
disaster focuses attention on cabin safety—and why instructions are important.”